
12 May 2025
Mark Leibler on his lobbying power israel-palestine, media, politics
In a speech he delivered back in 2018, Mark Leibler lays out how he exerts influence, from trying to block Bob Carr's efforts on recognition of a Palestinian State to watching ABC correspondent Sophie McNeill in order to change her coverage of the Middle East.

12 May 2025
Message from the editor
Welcome to a new week, a new pope, a new Cabinet and a new Opposition Leader. And for the first time, there are at least 57% women in the ALP Caucus and record numbers of women across the Federal Parliament.

12 May 2025
Shell-shocked voters of US allies choose stability over disruption
Rather than left- or right-leaning political parties, citizens in Singapore, Australia and Canada chose steady hands to navigate geopolitical turbulence.

Israel's war against Gaza
Media coverage of the war in Gaza since October 2023 has spread a series of lies propagated by Israel and the United States. This publication presents information, analysis, clarification, views and perspectives largely unavailable in mainstream media in Australia and elsewhere.
Download the PDF
12 May 2025
Can Albanese resist the temptation to fall for Trump’s flattery?
Without wishing to rain on the Australian Labor Party’s victory parade, when our prime minister was congratulated and praised by Donald Trump the day after Labor won the 2025 federal election, alarm bells should have been ringing to alert his advisers.

12 May 2025
Critical minerals offer a path to check US unilateralism
Trumpian trade policy promises a period of spasmodic aggression and persisting uncertainty.
12 May 2025
Recognising and embracing AI in research
Artificial intelligence and human endeavour can work together in harmony to reshape scholarly work.

12 May 2025
In memory of the Marshall Plan – a primer for Gen Z
The attack on Pearl Harbour led to an enormous volume of United States resources being committed to the war against Nazi Germany.

11 May 2025
The New Pope – Leo XIV
I thought it might be third time lucky, but I was wrong again. I backed the wrong horse as pope!

11 May 2025
Dutton’s election campaign rout lets RBA off the hook
Reserve Bank governor Michele Bullock must be breathing a quiet sigh of relief now the Albanese Government has been triumphantly returned to office. If you can’t think why she should be relieved, you’re helping make my point.

11 May 2025
In the age of the influencer, does the political backing of News Corp matter anymore?
This year’s federal election demonstrated that Australia’s media landscape has changed. Big players are no longer “kingmakers” in politics.

11 May 2025
At the ICJ, only US and Hungary back Israel starving Gaza
Thirty-seven states, the UN and international NGOs all condemned Israel’s denial of aid to the starving people of Gaza at the International Court of Justice in the first week of May.
Latest on Palestine and Israel

12 May 2025
Mark Leibler on his lobbying power
In a speech he delivered back in 2018, Mark Leibler lays out how he exerts influence, from trying to block Bob Carr's efforts on recognition of a Palestinian State to watching ABC correspondent Sophie McNeill in order to change her coverage of the Middle East.

11 May 2025
At the ICJ, only US and Hungary back Israel starving Gaza
Thirty-seven states, the UN and international NGOs all condemned Israel’s denial of aid to the starving people of Gaza at the International Court of Justice in the first week of May.
10 May 2025
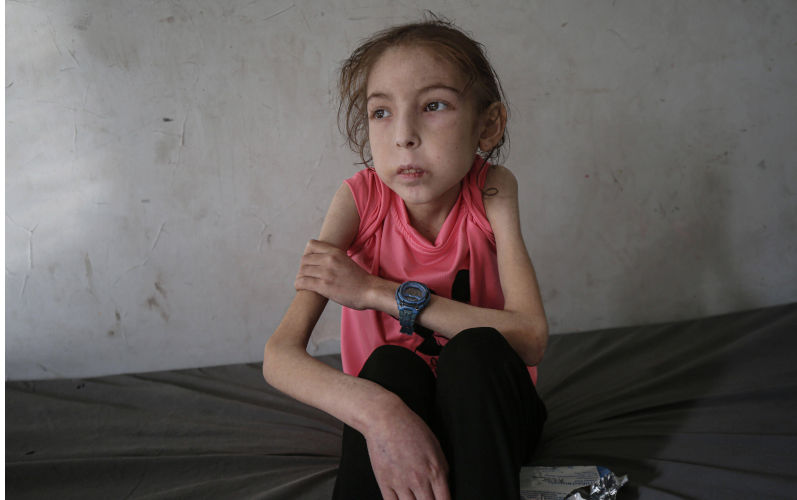
'This isn't me': Israeli war and healthcare collapse leave Gaza child unrecognisable
Under a tightening Israeli siege, Palestinian girl Rahaf Ayyad struggles with physical and emotional changes, as her mother fights for answers.

9 May 2025
Aid to Gaza: Moral and political dilemmas for Australia
Amidst preparations for a renewed assault intended to allow permanent Israeli occupation of Gaza, Israel and the United States are also about to establish a mechanism through which humanitarian aid will henceforth be distributed exclusively by private firms protected by the Israeli military.

9 May 2025
Re-elected Albanese Govt must condemn Israel's brutality and cut ties
On 5 May, the Israeli Parliament approved plans to annex and occupy Gaza. These plans have been discussed for months. This is a blatant mission to ethnically cleanse Gaza, advancing Israel’s colonial intentions to take over the territory and rid it of Palestinians.

9 May 2025
Zionist lawfare comes for Australian journalist
The Zionist federation of Australia should be recognised as a duplicitous and malicious actor in Australian society and politics.

8 May 2025
Mainstream media and distorted Palestine reporting
Australia’s mainstream media have ignored and distorted the genocide in Palestine. A recent Australians for Humanity forum, chaired by former SBS newsreader Mary Kostakidis, and featuring Margaret Reynolds, Stuart Rees and Peter Slezak, tackled the issues and discussed what needs to be done.

7 May 2025
Judaism and Zionism are not the same
No doubt about it. We live in a topsy-turvy world. How Kafkaesque can it get, when some of Zionism’s most fervent supporters have been politicians like Scott Morrison, Peter Dutton or — God help us — the Mad King of Mar-a-Lago?

Support our independent media with your donation
Pearls and Irritations leads the way in raising and analysing vital issues often neglected in mainstream media. Your contribution supports our independence and quality commentary on matters importance to Australia and our region.
DonateLatest on China

12 May 2025
Shell-shocked voters of US allies choose stability over disruption china, politics, world
Rather than left- or right-leaning political parties, citizens in Singapore, Australia and Canada chose steady hands to navigate geopolitical turbulence.

10 May 2025
China touts new law as foundation for private sector growth
A week after the passage of a law on China’s private economy, officials said the bill would unleash the potential of the non-state sector.

9 May 2025
Four World War II myths: Ignoring China, downplaying Russia’s role
As the 80th anniversary of the end of World War II approaches in 2025, the vital contributions of China and Russia remain largely overlooked by the West, just as they have always been.
More from Pearls and Irritations
Latest letters to the editor
Very helpful interpretation of the steady-state economy
Len Puglisi — 1 Balmoral Court Burwood East
The leopard can’t change its spots
Fiona Colin — Melbourne
Essential clarity from Sara Dowse
Stephanie Dowrick — Darwin 0800, NT
Tim Beal's articles in need of corrections
Craig Thomas — North Sydney








